CAR Cell Therapy
Step By Step
-
- Leukapheresis: First, native autologous T cells must be derived from the patient.
- This is similar to stem cell collection before autologous transplantation but involves selecting for CD3 (a T-cell marker) rather than CD34 (a stem cell marker).
- Because T cells normally circulate in our blood, no “mobilizing” medications such as granulocyte-colony stimulating factor (G-CSF) are needed.
- Bridging therapy (optional): treatments such as chemotherapy or radiation may be used to control the underlying malignancy, helping bridge patients through the several-week gap until they can receive autologous CAR-Ts.
- CAR transfer: autologous T cells must be turned into CAR-Ts that contain the CAR of choice.
- Retroviral or lentiviral vectors are typically used for transduction and integration of the CAR into T-cell DNA.
- CAR-T expansion and cryopreservation: Third, these T cells must then be expanded (coaxed to grow) ex vivo and cryopreserved for shipping back to the patient’s bedside
- Release testing and administration: Once the CAR-Ts are back at the patient’s institution, they must be tested. Among other things, release testing involves confirming the sterility of the product as well as the number of viable CAR-Ts. “Out-of-spec” CAR-T products are bags that don’t meet the strict parameters for infusion.
- Lymphodepletion (“LD”): often performed with fludarabine and cyclophosphamide, is to make “immunological space” for the incoming CAR-Ts.
- While bridging therapy is optional, lymphodepleting chemotherapy is not.
- Specifically, lymphodepletion alters in vivo cytokine and immune profiles (e.g., by reducing numbers of regulatory T cells and homeostatic cytokine “sinks”) to favor the expansion of incoming CAR-Ts.
- Monitoring for CRS: #todo
Cytokine Release Syndrome (CRS):
- CRS is generally thought to be driven by pro-inflammatory cytokines (in particular, IL-6) released by monocyte-lineage cells, rather than by the CAR-T cells themselves.
- CRS typically presents with fevers; in more severe cases hypotension or hypoxia may develop.
- The mainstay of CRS treatment is tocilizumab (“toci”), an IL-6 receptor antagonist and corticosteroids.
- While biomarker patterns (e.g., a rising ferritin or CRP) can help suggest CRS, CRS remains a clinical diagnosis.
- CRS can mimic bacterial sepsis, thus neutropenic fevers in a CAR-T therapy recipient must be treated w/ empiric antibiotics even if CRS is more strongly suspected.
- MSK CRS Protocol: [[MSK CRS Protocol.pdf]]
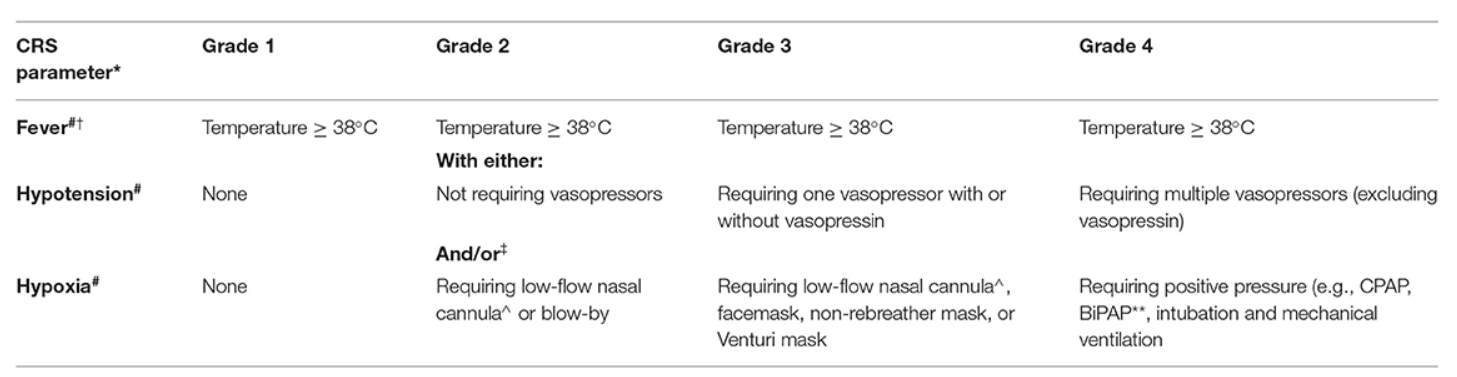
Other Adverse Effects:
- Immune effector cell-associated neurotoxicity syndrome (ICANS):
-
Thought to be driven by pro-inflammatory cytokines entering the central nervous system (CNS) even if the CAR-Ts themselves do not.
-
ICANS-related encephalopathy often presents with subtle word-finding difficulties but can progress to cerebral edema or seizures.
-
The ICE assessment is generally used to evaluate patients for neurological deficits following CAR-T therapy. Corticosteroids are the mainstay of ICANS treatment, and sometimes levetiracetam is used for prophylaxis.

-
- Hemophagocytic lymphohistiocytosis (HLH):
- Similar in presentation to CRS, but more likely to involve hyperferritinemia, coagulopathy, and transaminitis.
- Anakinra (an IL-1 antagonist) is often used preferentially here, along with high-dose corticosteroids.
- Cytopenias:
- Low blood counts and frequent transfusions are very common in the weeks following CAR-T therapy for many reasons: a heavily pre-treated bone marrow, underlying disease, lymphodepleting chemotherapy, CRS occurring within the marrow, etc.
- Growth factors, in particular G-CSF, are often avoided during the first one to two weeks after CAR-T administration to avoid the theoretical risk of exacerbating CRS.
- Infections: CAR-T recipients are highly susceptible to infections, in part due to low counts as explained previously.
- Additionally, on-target hypogammaglobulinemia (both with CD19-directed and BCMA-directed products) or the use of tocilizumab and corticosteroids can predispose patients to infections.
- Antimicrobial prophylaxis (including antifungal agents for high-risk patients) is often used for several months following CAR-T therapy, and some may also require IVIG.
- Other Organ Toxicities
- Cardiac (tachycardia, arrhythmias, heart block, heart failure)
- Respiratory (tachypnea, pleural effusion, pulmonary edema)
- Gastrointestinal (nausea, vomiting, diarrhea)
- Hepatic (ALT/AST/Bili elevations)
- Renal (AKI, decreased urine output)
- Dermatologic (rash)
- Hematologic (Coagulopathy, disseminated intravascular coagulation)
References:
Created at: periodic/daily/August/2023-08-01-Tuesday