Fluids - pH
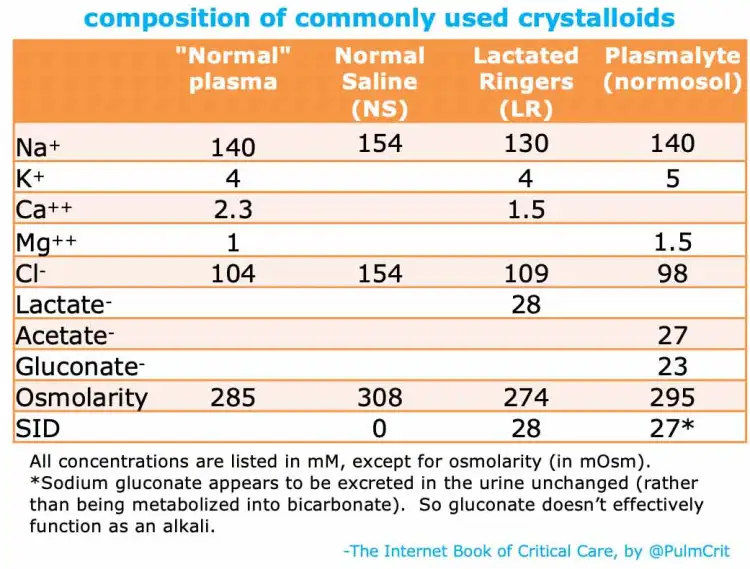
- Balanced crystaloids (lactated ringer's + normosol/plasmalyte) do not significantly impact serum pH. Normal Saline on the other hand induces acidosis.
Using Fluids To Correct Acid/Base Abnormalities
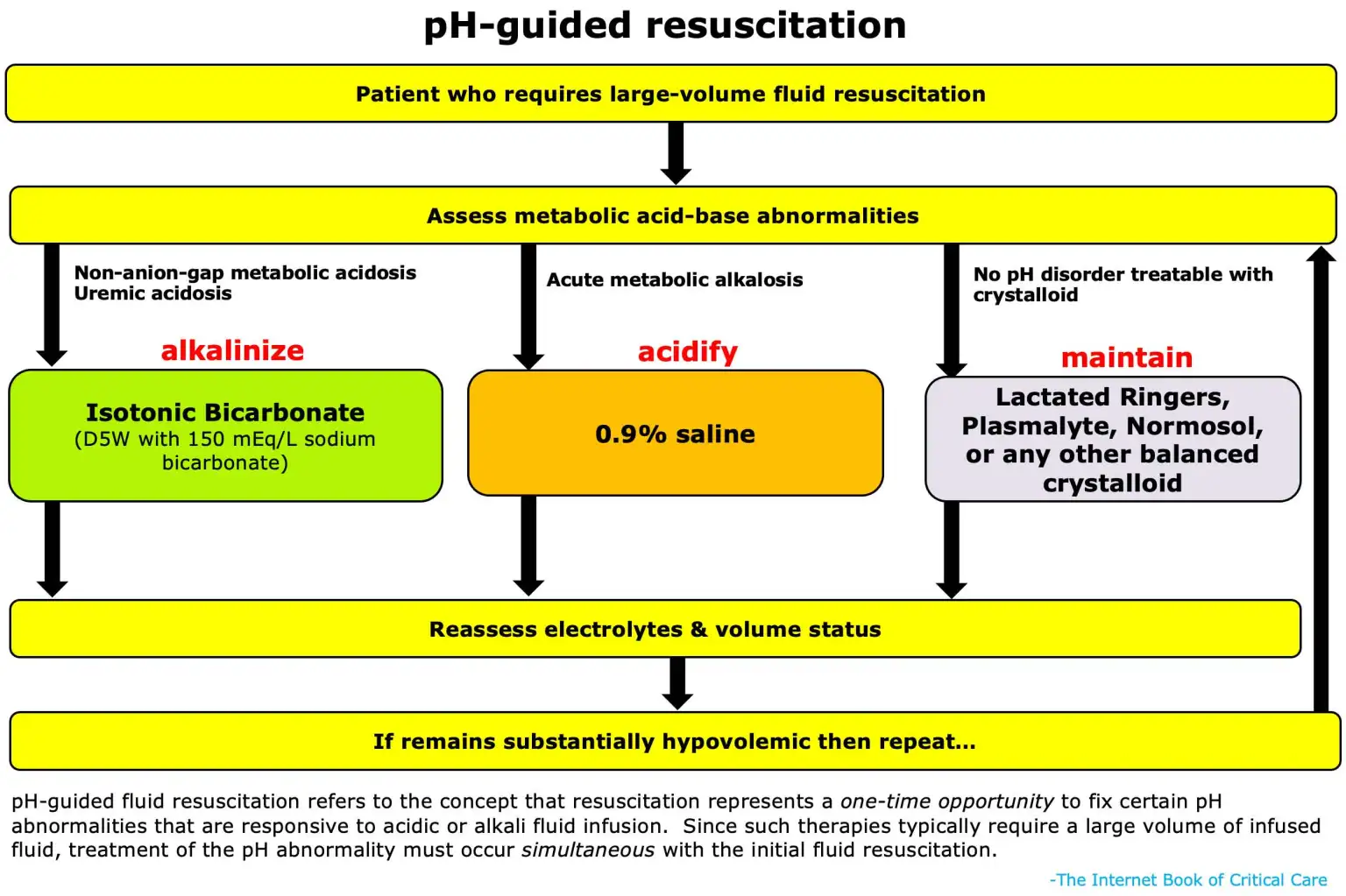
Crystaloids can only treat 3 major categories of acid/base abnormalities.
Treat with isotonic NaBicarb (150mEq/L) in D5W:
- NAGMA/Hyperchloremic Metabolic Acidosis: Physiologically this represents a deficit of bicarbonate from any number of causes (losses into diarrhea, urine, pancreatic fluid). Isotonic bicarbonate solutions can replace this lost bicarbonate and normalize pH.
- Normal kidneys will eventually regenerate this lost bicarb on their own, but we can accelerate this process with exogenous bicarb.
- Uremic acidosis (AG metabolic acidosis): While most AG acidosis are treated by correcting the underlying etiology (DKA, lactic acidosis, etc), uremic acidosis occurring in renal failure represents a failure of bicarb homeostasis by the kidneys.
- Bicarb helps to correct acidosis (HD indication) and hyperkalemia (HD indication), preventing pts with renal failure from progressing to HD
Treat with NS:
- Acute metabolic alkalosis: Etiologies such as contraction alkalosis, gastric acid losses (vomiting, NGT suctioning), and alkalai ingestion can be normalized using NS (as described below)
Mechanisms by which NS induces metabolic acidosis:
- Stewart's Acid Base Model - Strong Ion Difference:
- Stewart describes that electroneutrality must be preserved in body fluids (ie the concentration of + ions must equal that of - ions).
- If Cl- concentration increases, then there must be a balancing change to either decrease other negative ions or increase positive ions. This occurs by the nature of the body's major buffer system, the bicarbonate buffer. If Cl- increases then relatively H+ must increase/HCO3- must decrease
- Renal blood flow:
- In a healthy patient, the macula densa senses chloride concentration in the filtrate delivered to the distal nephron. If too much chloride is delivered this suggests the GFR is too high, overwhelming the transport mechanisms which resorb chloride. Thus the macula dense secretes local mediators which constrict the afferent arteriole and decrease glomerular pressure and GFR.
- The kidney maintains acid-base balance by excreting acid and resorbing bicarbonate
- Elevated serum chloride leads to increased chloride in the filtrate of the nephron. When all of this chloride cannot be resorbed, increased chloride is delivered to the macula densa which causes decreased glomerular filtrate.
- If GFR decreases, bicarbonate continues to be resorbed, but the kidney cannot excrete sufficient acid, leading to acidosis
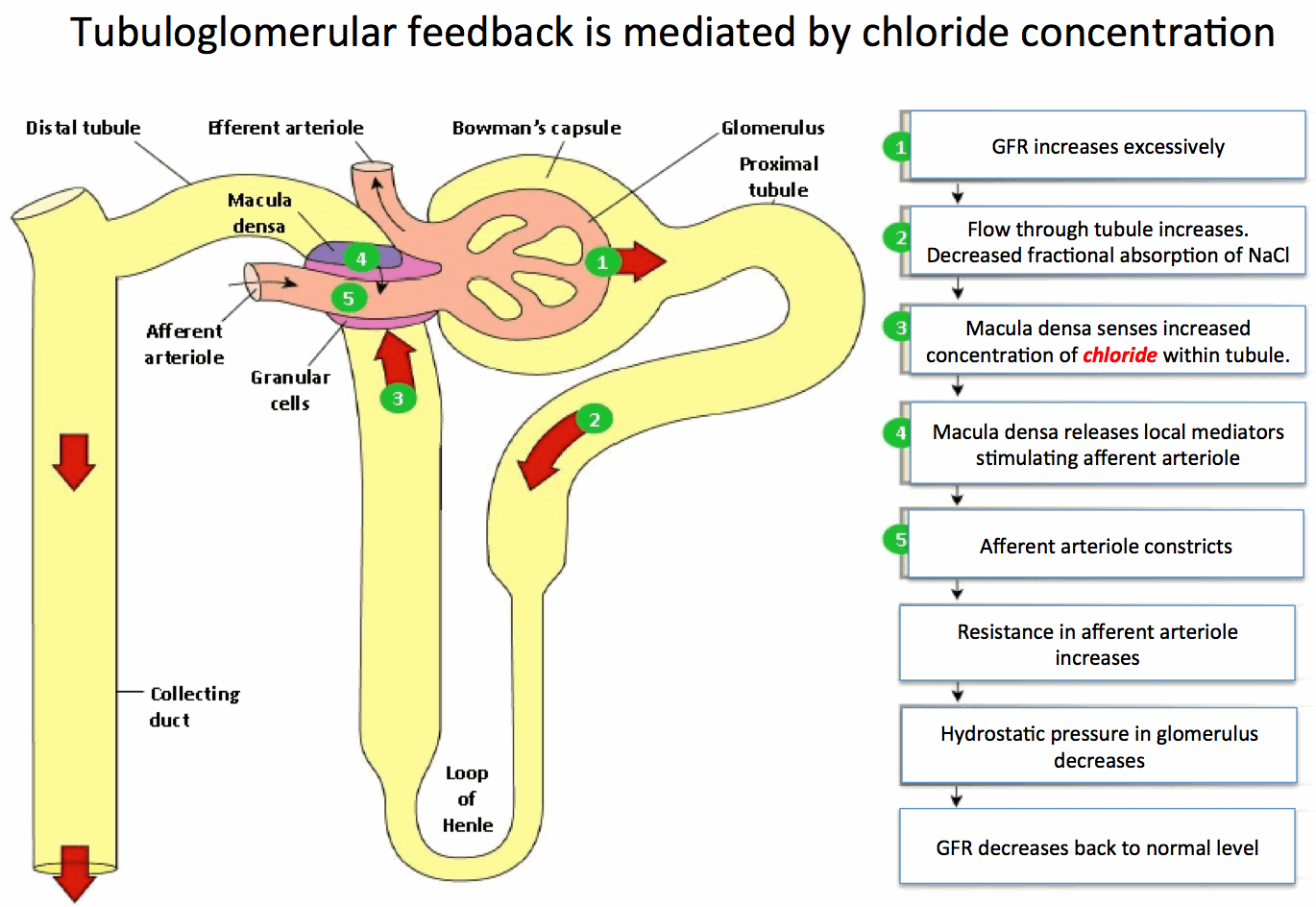
- Renal compensation for chloride load:
- There may be an impact of increased chloride in the glomerular filtrate on bicarbonate resorption but the mechanism for this is not clearly documented.
References:
Created on: Thursday 04-25-2024